February 2023
News
In this issue
| PBS Changes to Morphine and Oxycodone oral solution – 1 February 2023 |
|
| What's new in Fred Dispense? |
|
| Your new customer support service |
|
| Reminder: New South Wales S8 and S4 drugs require patient's date of birth |
|
| Reminder: Services Australia PRODA B2B Device expiry |
|
| This Month's PBS Changes |
|
PBS Changes to Morphine and Oxycodone oral solution – 1 February 2023
The Department of Health is making changes to the listing of the below-mentioned products on the PBS to allow pharmacists to dispense volumes smaller than a whole bottle at PBS subsidised prices.
-
Oral solution containing morphine hydrochloride trihydrate 2 mg per mL, 200 mL
-
Oral solution containing morphine hydrochloride trihydrate 5 mg per mL, 200 mL
-
Oral solution containing morphine hydrochloride trihydrate 10 mg per mL, 200 mL and
-
Oral solution containing oxycodone hydrochloride 1 mg per mL, 250 mL
The changes being implemented mirror those made on 1 August 2022 to the listing of oral solution containing hydromorphone hydrochloride 1 mg per mL, 1 mL following consultation with key stakeholders representing prescribers, pharmacists, pharmacy dispensing software, electronic Schedule 8 drug registers, and state and territory health authorities.
|
Amendments to the PBS listing of Morphine oral solution Change |
||
|---|---|---|
|
Current |
Amendments from 1 February 2023 |
|
|
Legislative Instrument form descriptions |
Oral solution containing hydromorphone hydrochloride 2 mg per mL, 200 mL |
Oral solution containing hydromorphone hydrochloride 2 mg per mL, 1 mL |
|
Oral solution containing morphine hydrochloride trihydrate 5 mg per mL, 200 mL |
Oral solution containing morphine hydrochloride trihydrate 5 mg per mL, 1 mL |
|
|
Oral solution containing morphine hydrochloride trihydrate 10 mg per mL, 200 mL |
Oral solution containing morphine hydrochloride trihydrate 10 mg per mL, 1 mL |
|
|
Pharmaceutical Item value |
1 unit (ie. bottle) |
200 units (ie. millilitres) |
|
Pack Qty |
1 |
200 |
|
Pricing Qty |
1 |
200 |
|
Max Qty Units |
1 |
200 |
|
Amendments to the PBS listing of Oxycodone oral solution Change |
||
|---|---|---|
|
Current |
Amendments from 1 February 2023 |
|
|
Legislative Instrument form descriptions |
Oral solution containing oxycodone hydrochloride 1 mg per mL, 250 mL |
Oral solution containing oxycodone hydrochloride 1 mg per mL, 1 mL |
|
Pharmaceutical Item value |
1 unit (ie. bottle) |
250 units (ie. millilitres) |
|
Pack Qty |
1 |
250 |
|
Pricing Qty |
1 |
250 |
|
Max Qty Units |
1 |
250 |
Pharmacy action
When supplying these prescriptions as originals or repeats, which were written prior to but supplied on or after 1 Feb 2023, pharmacies must update the quantity representing the number of mL’s as applicable (remembering to allow for smaller volumes) from what was previously represented as a pack quantity of 1.
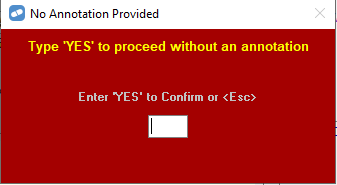
Example: PBS Scripts for one bottle of hydromorphone oral solution written prior to 1 February 2023 and dispensed after 1 February 2023 should be claimed with quantity 200 (for morphine hydrochloride trihydrate) or 250 (for oxycodone hydrochloride). An annotation may be required when increasing the quantity for electronic script.


However, you can proceed without the annotation by entering YES to the following prompt
For more information please see the announcement, Changes to PBS listing of morphine and oxycodone oral solutions , from the Department of Health.
What's new in Fred Dispense?
New indicator on dispense label and coding sticker for ePrescriptions
Electronic prescriptions will now display a new indicator ('E') on dispense labels and coding stickers. The 'E' prints to the right of the number of repeats.
Click the thumbnail below to see a larger image.
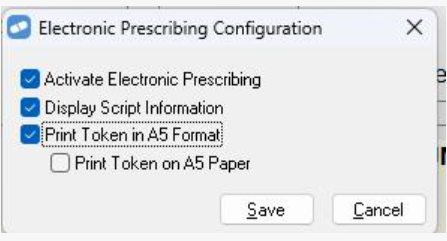
Option to print ePrescription repeat tokens in A5 format
You now have the option to print ePrescription repeat tokens in A5 format. To turn on this option:
-
From the Setup menu, select eHealth Configuration > Electronic Prescribing Configuration.
-
Select the Print Token in A5 Format checkbox and Save.
With this option selected, repeat ePrescription tokens will continue to print on the same printer as currently configured, but will be rotated and reduced in size to use half a sheet of A4 paper.
See Print A5 ePrescription repeat tokens for more information.
Your new customer support service
We are excited to announce your new look customer support service that will make it easier and faster for us to respond to your needs.
What's New?
-
Fred Customer Portal: Create a service request, view & manage your requests, search the knowledgebase
-
Live Chat: Interact with a Fred Help consultant to ask quick questions
What's Changed?
-
Improved Fred Service Levels (including Saturday)
-
Extended business hours
-
Outside of business hours, emergency only Sunday
Reminder: New South Wales S8 and S4 drugs require patient's date of birth
From 1 November 2022, pharmacists in New South Wales must record the patient's date of birth for all S8 and S4 Drugs.
Fred Dispense now prompts for date of birth when an S8 or S4 drug is dispensed in New South Wales. From 1 February 2023, this prompt can no longer be bypassed. See Providing Date of Birth when Dispensing S8 Drugs for more details.
See also Requirement for patient date of birth on prescriptions and in dispensing records on the NSW Health website.
Reminder: Services Australia PRODA B2B Device expiry
To avoid disruption to your Services Australia PBS Online claiming, you’ll need to extend your B2B Device in your PRODA account before it expires.
You’ll need to extend your B2B device with PRODA at least every 6 months.
Fred Dispense will warn you of the impending expiry at the following time intervals:
-
30 days from expiry
-
14 days from expiry
-
Every day from 7 days to the expiry day.
For more information on how to do this, refer to: Managing B2B Devices in PRODA - PRODA (Provider Digital Access) on the Services Australia website or call Services Australia on 1800 700 199.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.
