August 2022
News
In this issue
| PBS Changes to Hydromorphone oral solution – 1 August 2022 |
|
| Get Help with Fred Dispense |
|
| Reminder: Services Australia PRODA B2B Device expiry |
|
| This Month's PBS Changes |
|
PBS Changes to Hydromorphone oral solution – 1 August 2022
The Department of Health is making changes to the listing of oral solution containing hydromorphone hydrochloride 1 mg per mL, 473 mL (hydromorphone oral solution) on the PBS to allow pharmacists to dispense volumes smaller than a whole bottle at PBS subsidised prices.
The changes being applied on 1 August 2022 are detailed in the table below and will apply to the two brands of hydromorphone oral solution currently subsidised by the PBS – Hikma and Hydromorphone hydrochloride oral solution, USP (Medsurge) under item codes 12582F, 12559B and 12565H.
| Amendments to the PBS listing of hydromorphone oral solution Change | ||
|---|---|---|
| Current | Amendments from 1 August 2022 | |
|
Legislative Instrument form descriptions |
Oral solution containing hydromorphone hydrochloride 1 mg per mL, 473 mL |
Oral solution containing hydromorphone hydrochloride 1 mg per mL, 1 mL |
|
Pharmaceutical Item value |
1 unit (ie. bottle) |
473 units (ie. millilitres) |
|
Pack Qty |
1 |
473 |
|
Pricing Qty |
1 |
473 |
|
Max Qty Units |
1 |
473 |
Pharmacy action
When supplying these prescriptions as originals or repeats, which were written prior to but supplied on or after 1 Aug 2022, pharmacies must update the quantity representing the number of mL’s as applicable (remembering to allow for smaller volumes) from what was previously represented as a pack quantity of 1.
Example : PBS Scripts for one bottle of hydromorphone oral solution written prior to 1 August 2022 and dispensed after 1 August 2022 should be claimed with quantity 473. An annotation may be required when increasing the quantity for electronic script.


However, you can proceed without the annotation by entering YES to the following prompt
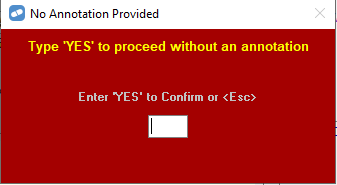
Services Australia will reimburse pharmacies based on listing price on the Date of Supply of the product. Scripts of hydromorphone oral solution dispensed prior to 1 August 2022 will not be impacted and should remain as quantity ‘1’.
FRED advises, scripts submitted prior to 1 August 2022 should not be edited or resubmitted as this pack size update may have unknown flow on effects to POS, DD Book and reporting. Note that the payment from PBS for these scripts will not be affected, however estimated figures from Dispense reports may be inaccurate upon edits, affecting reconciliation efforts.
For more information please see the announcement, Changes to PBS listing of hydromorphone oral solution , from the Department of Health.
Catchup with the Fred team at PSA22 – visit us on Stand 48
Get Help with Fred Dispense
Fred Dispense Online Help contains information about how to use Fred Dispense, including step-by-step procedures and short videos. You can access Online Help:
-
In Fred Dispense, select Help > Fred Help Centre.

-
Alternatively, while viewing the monthly newsletter, you can browse from the menu (A) at the top of the screen, or enter your search terms in the Search box (B).

Reminder: Services Australia PRODA B2B Device expiry
To avoid disruption to your Services Australia PBS Online claiming, you’ll need to extend your B2B Device in your PRODA account before it expires.
You’ll need to extend your B2B device with PRODA at least every 6 months.
Fred Dispense will warn you of the impending expiry at the following time intervals:
-
30 days from expiry
-
14 days from expiry
-
Every day from 7 days to the expiry day.
For more information on how to do this, refer to: Managing B2B Devices in PRODA - PRODA (Provider Digital Access) on the Services Australia website or call Services Australia on 1800 700 199.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.
