August 2019
News
In this issue
| New Prescriber Details Mismatch Warning |
|
| Enhanced Patient Details Mismatch Warning |
|
| SafeScript and Fred Dispense for Victorian Pharmacies |
|
| Are you eHealth ready? |
|
| This Month's PBS Changes |
|
New Prescriber Details Mismatch Warning
The Prescriber Details Mismatch warning displays when you change the prescriber details on an electronic script (eScript) that you've scanned. The warning will alert you that you have changed the prescriber and enable you to correct the change by using the prescriber information contained in the eScript.
If you choose instead to use the prescriber details in Fred Dispense, then the result will be a broken eScript repeat chain as Fred Dispense will then issue a new electronic script barcode to ensure the accuracy of electronic records is maintained.
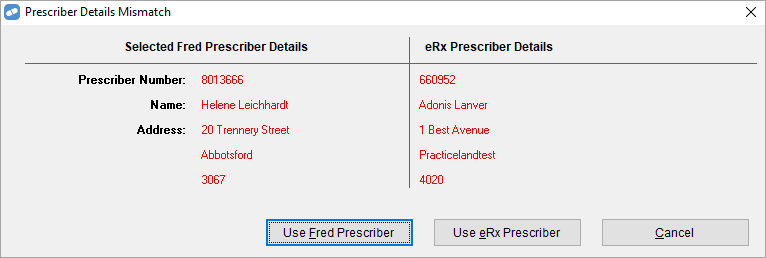
For more details, see Prescriber Details Mismatch Warning.
Enhanced Patient Details Mismatch Warning
In the October 2018 release, a Patient Details Mismatch Warning was added to alert you when there is a mismatch between patient details contained in the select Fred patient and the patient details contained in the electronic (eScript) record.
This warning message has now been enhanced:
-
to alert if DOB, Gender, Medicare or IHI are missing in Fred Dispense—previously, the alert wasn't displayed in these scenarios.
The above change may result in an increase in frequency of the warning, but will enable you to ensure better accuracy of your Fred Dispense patient data.
- to give you the option to select details from the eScript record to automatically update your record in Fred Dispense.
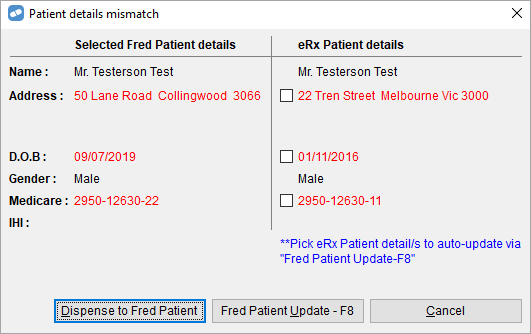
To update the Fred Patient record with the eRx Patient details, select the relevant checkboxes, and then select Fred Patient Update - F8.
Selecting Dispense to Fred Patient will still allow you to continue with the currently selected Fred Dispense patient record as in the previous version.
For more details, see Patient Details Mismatch Warning.
SafeScript and Fred Dispense for Victorian Pharmacies
SafeScript real-time prescription monitoring system is already saving lives and keeping patients with prescription medication addictions safe from harm.
SafeScript came online in Western Victoria at the start of October 2018 and is now being rolled out across the state.
Get Started with SafeScript
Victorian pharmacies wishing to use SafeScript must first register with the Victorian Department of Health and Human Services
Once the registration process is complete, activate SafeScript in Fred Dispense.
- Go to Setup > eHealth Configuration > SafeScript Configuration.
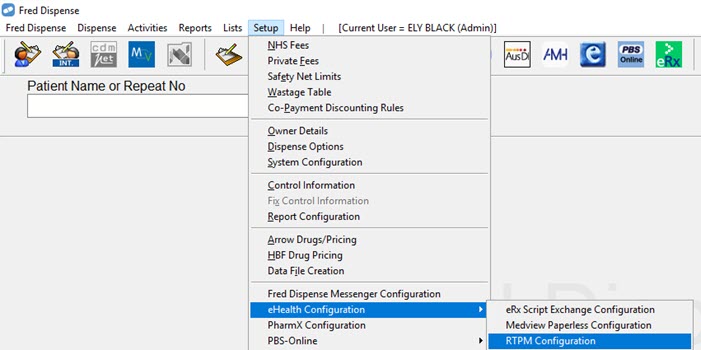
- Select Activate SafeScript.

- Click Save.
- When you dispense S8 or Victorian monitored drugs, you must enter the patient's Date of Birth.
- SafeScript returns a notification when you navigate to the Pharmacist initials field.
For more information, refer to SafeScript in Fred Dispense.
In preparation for state wide rollout, Victorian pharmacies providing services to hospitals (specifically inpatient) will be able to flag a script as being dispensed as an inpatient script with the keyboard shortcut CTRL+SHIFT+I. See Dispense a script for an inpatient for more details.
This information is important for future rules to be implemented in SafeScript. All monitored drug dispensing history is forwarded to SafeScript with this inpatient information included.
Are you eHealth ready?
Our industry is embarking on significant digital advances which have or will impact the way your pharmacy operates.
Visit the eRx Script Exchange team on stand 34 at PSA19 to discuss how you can prepare your pharmacy today.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site:
Please note that the Government provides this information under embargo to Fred IT Group to enable next month’s PBS changes to be incorporated into your drug file. We have provided this information in this newsletter to prepare you for the changes, maximising patient availability/access and enabling you to adjust your stock accordingly. A condition of the embargo and in the interests of the quality use of medicines is that the information must not be released to the public or otherwise distributed prior to 1 August 2019.
