September 2024
News
In this issue
| Errata to the 1 September 2024 PBS Schedule |
|
| 60 Day Dispensing of PBS Medicines |
|
| Pharmacy Connect & PA National Conference | 5-7 Sep |
|
| International Harmonisation of Ingredient Names (IHIN) |
|
| This Month's PBS Changes |
|
Errata to the 1 September 2024 PBS Schedule
PBS have published an erratum containing the following errata to the September 2024 PBS Schedule:
(1) This Erratum corrects the clinical criteria for new Migalastat item 14573B in the 1 September 2024 Schedule.
(2) This Erratum corrects the DPMQ for CLAVULIN-125F (GlaxoSmithKline, Canada) brand of Amoxicillin with clavulanic acid in the 1 September 2024 Schedule.
For more information, see Errata to PBS Schedule.
60 Day Dispensing of PBS Medicines
For general information on 60 day dispensing, please visit https://www.health.gov.au/cheaper-medicines
From 1 September 2024, medicines in stage 3 will have additional PBS Item Codes added to cater for 60 day dispensing.
A list of medications available for 60 day dispensing will be available here https://www.health.gov.au/our-work/60-day-dispensing/pbs-medicines-current-item-codes from 1 September 2024.
There will be an additional PBS item code for a drugs that are listed under 60 days dispensing.
For example, searching for 'Felodur 10', will now produce two search results instead of one.
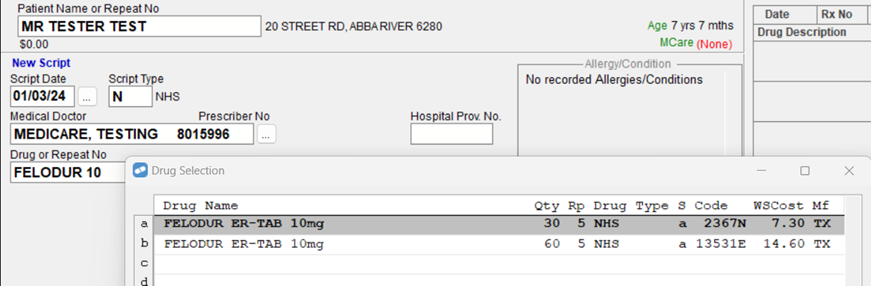
You will then select the applicable PBS Item Code based on the quantity on the prescription. This is a process that you will already be familiar with today for drugs that have multiple PBS Item Codes e.g. Nolvadex 20mg Tablets, Clexane 100mg/mL Syringe, Brufen 400mg Tablets
See also: Set NHS/OTC Prices for Different Quantities of an Under Co-Payment NHS Item
Pharmacy Connect & PA National Conference | 5-7 Sep
We hope to see you and your team at the upcoming Pharmacy Connect and Pharmacy Assistant Conference in Sydney. Tickets to the trade exhibition are free.
Visit Fred and MedView on stands 67 & 68 to see a live demo of NEW MedView Flow Digital Baskets . See how you can revolutionise your pharmacy workflow!

International Harmonisation of Ingredient Names (IHIN)
In 2017, the Therapeutic Goods Administration (TGA) updated over 200 active ingredient names in Australia to align with international standards, a program known as the International Harmonisation of Ingredient Names (IHIN). This introduced some new ingredient names to Australia through dual labelling, which requires both old and new names to be displayed on medicine labels.
Upcoming Changes in Fred Dispense
Dual labelling will be switched off in Fred Dispense in the October update for all ingredients except those specified for ongoing dual labelling. This change means that Fred Dispense will transition to displaying only the new names. However, certain ingredients will continue with dual labelling indefinitely to maintain both the old and new names.
Dual Labelling Transition Period
Dual labelling for most ingredient names ended on 30 April 2023, transitioning to displaying only the new names on labels. After this date, labels can either show the new name only or continue with both names while updates are made. Medicine sponsors have until 30 April 2026 to complete these updates. For example, medicines labelled as ‘lidocaine (lignocaine)’ will need to be updated to show ‘lidocaine’ only.
The transition period for most dual-labelled ingredients started on 1 May 2023 and ends on 30 April 2026, after which all medicines must show the sole name. Ingredient names that are changing to show new names only are found in TGA’s list of affected ingredient names marked as ‘Dual labelling - until 30 April 2023’ .
Some ingredients will have an extended dual labelling period until 2025 to give health professionals more time to adapt. Until 30 April 2025, labels must show both names for:
-
dosulepin (dothiepin) hydrochloride
-
hydroxycarbamide (hydroxyurea)
-
tetracaine (amethocaine)
-
tetracaine (amethocaine) hydrochloride
-
trihexyphenidyl (benzhexol) hydrochloride
The transition period for these starts on 1 May 2025 and ends on 30 April 2028, after which only new names will be shown.
Other ingredients will retain dual labelling indefinitely, including:
-
alimemazine (trimeprazine) tartrate
-
mercaptamine (cysteamine)
-
mercaptamine (cysteamine) bitartrate
-
mercaptamine (cysteamine) hydrochloride
-
Mycobacterium bovis (Bacillus Calmette and Guerin (BCG) strain).
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.