August 2023
News
In this issue
| 60 Day Dispensing of PBS Medicines |
|
| Intercode substitution for Colestyramine 4g powder for oral liquid |
|
| August 2023 Update: Budesonide + Formoterol (Duoresp, Biresp) 11301T Claiming. |
|
| Are you attending PSA23? |
|
| Changing Approval Number? |
|
| Reminder: Services Australia PRODA B2B Device expiry |
|
| This Month's PBS Changes |
|
60 Day Dispensing of PBS Medicines
For general information on 60 day dispensing, please visit https://www.health.gov.au/our-work/60-day-dispensing
From 1 September 2023, medicines in stage 1 will have additional PBS Item Codes added to cater for 60 day dispensing.
A list of medications available in stage 1 is available here https://www.health.gov.au/our-work/60-day-dispensing/pbs-medicines-current-item-codes
When searching for drugs with a prescription date of 1 September 2023 or after, you will now see an additional PBS item code.
For example, searching for 'Felodur 10', will now produce two search results instead of one.
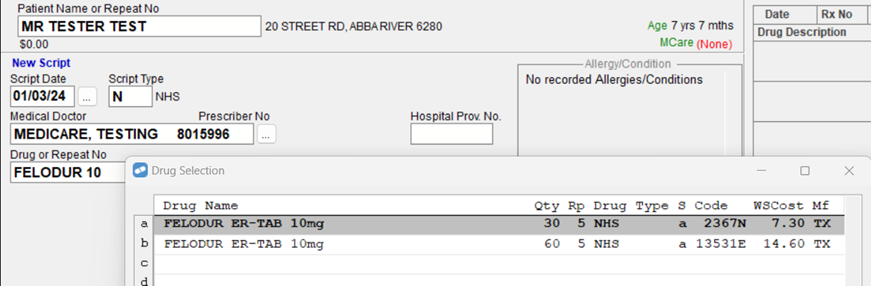
You will then select the applicable PBS Item Code based on the quantity on the prescription. This is a process that you will already be familiar with today for drugs that have multiple PBS Item Codes e.g. Nolvadex 20mg Tablets, Clexane 100mg/mL Syringe, Brufen 400mg Tablets
You will not see the additional PBS Item Codes if the prescription date is before 1 September 2023
Intercode substitution for Colestyramine 4g powder for oral liquid
As denoted in the Aug 2023 PBS Schedule :
Pharmaceutical benefits that have a 50 x 2 pack size equating to 100 sachets and a 30 x 3.334 pack size equating to 100 sachets are equivalent for the purposes of substitution.
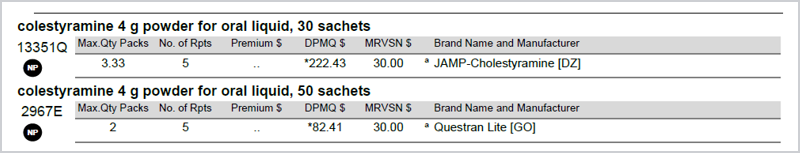
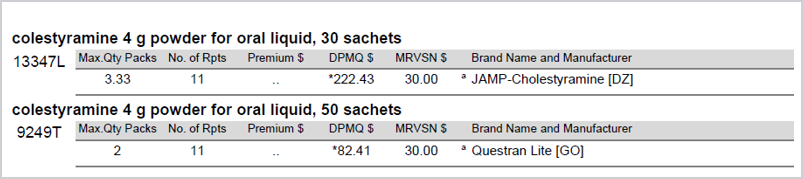
Due to the complexity of different pack sizes and achieving accurate DPMQ across both brands, substitution between the following PBS item codes will not be available in Fred Dispense via the usual GS shortcut.
13351Q and 2967E
13347L and 9249T
Instead, search and select the brand required when dispensing under these PBS codes.
August 2023 Update: Budesonide + Formoterol (Duoresp, Biresp) 11301T Claiming.
In July 2023, the PBS Schedule included changes to the pack size and max quantity of budesonide 400 microgram/actuation + formoterol (eformoterol) fumarate dihydrate 12 microgram/actuation powder for inhalation, 2 x 60 actuation (PBS item code 11301T) from 1 to 2 in July 2023 to allow for substitution with brands marketed as a single-inhaler pack (13258T).
This had unintended consequences for prescriptions written before 1st July and supplied after July, where a rejection R144 was returned for quantity of 2 for item 11301T. Services Australia implemented a temporary approval number “J2023BF” to allow an ‘increased quantity’ of 2 to be submitted and claimed for this item until the data was corrected in the August 2023 PBS Schedule.
In a late notice on 28th July 2023, PBS have announced that they will be continuing the arrangement of the temporary authority code J2023BF until 30 June 2024.
https://www.pbs.gov.au/info/news/2023/07/update-substitution-of-budesonide-formoterol-listings
Excerpt from the web page:
How should prescriptions written for item 11301T prior to 1 July 2023 be handled?
Prescriptions for budesonide 400 microgram/actuation + formoterol fumarate dihydrate 12 microgram/actuation powder for inhalation written prior to 1 July 2023 will be reimbursed correctly when:
-
PBS prescriptions and/or repeats for item 11301T with a Max Quantity of 1 written prior to 1 July 2023 and dispensed after 1 July 2023 should be claimed with a quantity of 2, using the temporary authority code J2023BF.
-
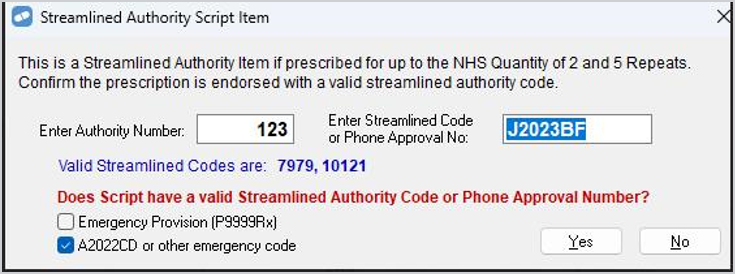
New scripts/repeats for 11301T with script date prior to 1 July 2023, ensure dispensed quantity is 2 and enter J2023BF in the Streamline Code/ Phone Approval Number field.
Make sure that the A2022CD or other emergency code checkbox is selected as shown below.
-
New scripts for 11301T with script date on or after 1 July 2023, emergency code not required. Please enter valid streamline code on script.
Are you attending PSA23?
Explore the role of digital health in optimising everyday pharmacy practice and improving patient health outcomes.
Unlock the power of digital health
Chrysa Giannellis, Managing Partner, Hummingbird Pharmaceutical Consulting
Sunday, 30 July 2023, 9.50 AM – 10.30 AM
Changing Approval Number?
To avoid any interruption in trading at full capacity, it is important you give us plenty of advanced notice.
We have prepared this quick guide to Changing Approval Number for current and new owners to ensure a smooth business transition.
Reminder: Services Australia PRODA B2B Device expiry
To avoid disruption to your Services Australia PBS Online claiming, you’ll need to extend your B2B Device in your PRODA account before it expires.
You’ll need to extend your B2B device with PRODA at least every 6 months.
Fred Dispense will warn you of the impending expiry at the following time intervals:
-
30 days from expiry
-
14 days from expiry
-
Every day from 7 days to the expiry day.
For more information on how to do this, refer to: Managing B2B Devices in PRODA - PRODA (Provider Digital Access) on the Services Australia website or call Services Australia on 1800 700 199.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.

