April 2021
News
In this issue
| Embedded MedView Flow in Fred Dispense |
|
| Check on Service Performance Status |
|
| MySL is coming soon - Are you ready? |
|
| What's New in Fred Dispense? |
|
| PBS Changes to Adalimumab – 1 April 2021 |
|
| This Month's PBS Changes |
|
Embedded MedView Flow in Fred Dispense
Wondering what this new addition to your dispense screen is?
It's the NEW embedded MedView Flow, and not only does it makes dispensing ePrescriptions easy, it's also ready to use right now!
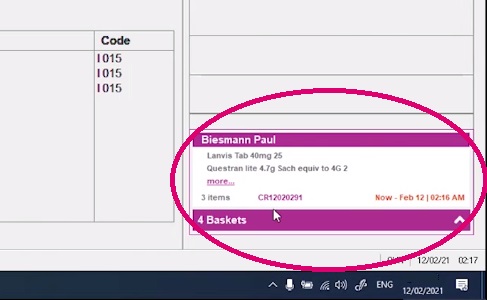
See how MedView Flow embedded works
Watch the DEMO
View help topic: ePrescribing in Fred Dispense
Looking for more ePrescription Resources?
The following resources will assist with your ongoing preparations for ePrescriptions.
WEBINAR VIDEOS
- The next phase - MySL and Dispensing ePrescriptions
- Beyond the Token Webinar Videos
- Dispensing ePrescriptions Webinar Videos
RESOURCES
Check on Service Performance Status
Our platforms are continuously monitored for outages and performance issues. Should there be any interruptions in service—for example to MedView Flow, we’ll provide status updates on the Service Performance Status page of our website.
MySL is coming soon - Are you ready?
My Script List (MySL) is an optional service which allows patients and their chosen healthcare providers to view a list of their available prescriptions.
MySL is in pilot throughout March 2021 and will be available widely in April (date to be confirmed). In order to prepare, Fred Dispense users should check that their Terminal ID has been added to MedView Flow.
-
In Fred Dispense, you can see the Terminal ID at the top of the screen.
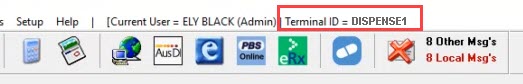
The Terminal ID is only visible if ePrescribing has been activated in Fred Dispense. If you cannot see Terminal ID on the top of the screen, select Help > About and then locate the Machine Name field. The value listed here is the Terminal ID.
For addition information, refer to Activate ePrescribing in Fred Dispense
This video contains audio. Headphones are recommended.
-
Select the User Account menu, then select Terminal Settings.

-
Enter the Terminal ID and Save.
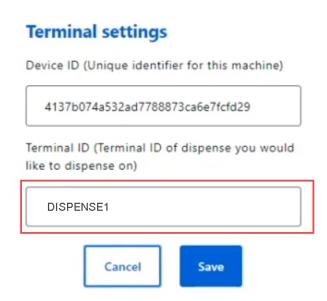
The Terminal ID must be entered into MedView Flow exactly as it appears in Dispense. Any variation in the ID settings will prevent MedView Flow and Fred Dispense from communicating.
The Device ID is automatically populated by MedView Flow.
What's New in Fred Dispense?
Fred Dispense is now able to process ePrescriptions with Prescribed Quantity of zero, if the Extended Prescribed Quantity field is populated.
Example
| Drug | Prescribed Quantity | Extended Prescribed Quantity |
|---|---|---|
| Eleuphrat Ointment 0.05% | 0 | 2*15g |
When you dispense a script in this scenario, a new Confirm Quantity popup displays, giving you the opportunity to confirm or update the correct quantity to dispense.

PBS Changes to Adalimumab – 1 April 2021
SUBSTITUTION - BIOSIMILLARS and NEW HUMIRA PRESENTATION
The Pharmaceutical Benefits Scheme (PBS) for adalimumab will be amended on 1 April 2021 with 4 biosimilars listed alongside the originator brand Humira®.
A new reduced volume formulation of Humira will also be PBS listed. This new formulation delivers and equivalent dose to existing formulation (20mg and 40mg) and is ‘a-flagged’ with both original formulations as well as the new biosimilar products.
Pharmacy action
Pharmacists may substitute brands that are ‘a-flagged’, in consultation with the patient. When dispensing, attention must be paid to the selection of the correct PBS item code to ensure the correct product is claimed. Due to the complexities introduced by the number of PBS item codes for adalimumab, substitution between item codes via the usual GS shortcut in Fred may be limited.
Fred advises using the streamline search functionality (where script is streamlined authority) as well as to exercise caution when selecting PBS item codes to ensure claims are successful. For any assistance selecting the correct PBS item code on the prescription, please contact Services Australia.
CHANGES TO PACK SIZES
Humira® remains available in pack sizes of 2, 4 or 6 units (either 2, 4 or 6 syringes or 2, 4 or 6 pens). Each biosimilar brand of adalimumab is available in pack sizes of 2 units (either 2 syringes or 2 pens). The PBS listings will allow various max quantities of 2, 4 or 6 units to be prescribed. The packs must therefore be dispensed as a single pack or in multiples of 1, 2 or 3 to make up the prescribed maximum quantity.
Prior to 1 April, the PBS listed Humira® pack sizes as ‘1 unit’. With the inclusion of variable pack sizes for the biosimilar brands, these pack sizes have been amended to 4 and 6 units respectively. Note only the pack sizes for 4 and 6 unit listings have been amended for April 2021. There has been no changes to pack size for 2 units.
The 11 PBS items below which prior to April had a Maximum Quantity (MQ)/pack size = 1 which will change in MQ/pack size effective 1 April 2021 are as follows:
- 8961P (MQ= 6)
- 8962Q (MQ= 6)
- 9186L (MQ= 6)
- 9187M (MQ= 6)
- 10397F (MQ= 6)
- 10404N (MQ= 6)
- 10945C (MQ= 6)
- 10972L (MQ= 6)
- 11132X (MQ= 6)
- 11133Y (MQ= 4) [2 Repeats]
- 11137E (MQ= 4) [5 Repeats]
Pharmacy action
-
New scripts/repeats
When supplying these prescriptions as originals or repeats, which were written prior to, but supplied after 1 April 2021, pharmacies must update the quantity representing the units (pens/syringes) supplied to 4 or 6 as applicable (remembering to allow for broken packs) from what was previously represented as a pack quantity of 1.
-
Edits and resubmissions
When editing and resubmitting a claim for items supplied and originally claimed prior to 1 April 2021, quantity should remain as ‘1’ as PBS Online will assess and pay as per the March 2021 DPMQ.
FRED advises that, if possible, do not edit any scripts submitted prior to 1 April 2021 as the pack size update may have flow-on effects to Point of Sale and reporting. Note that payment from PBS for these scripts will not be affected—however, estimated figures from Dispense Reports may be inaccurate upon edits, affecting reconciliation efforts.
For more information on these changes, please see the following announcements from:
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site:
Please note that the Government provides this information under embargo to Fred IT Group to enable next month’s PBS changes to be incorporated into your drug file. We have provided this information in this newsletter to prepare you for the changes, maximising patient availability/access and enabling you to adjust your stock accordingly. A condition of the embargo and in the interests of the quality use of medicines is that the information must not be released to the public or otherwise distributed prior to 1 April 2021.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.