October 2024
News
In this issue
| Ready to discover a smarter way to handle scripts in your pharmacy? |
|
| International Harmonisation of Ingredient Names (IHIN) |
|
| Additional Community Supply Support (ACSS) Payments |
|
| What's New in Fred Dispense |
|
| This Month's PBS Changes |
|
Ready to discover a smarter way to handle scripts in your pharmacy?
We’re proud to bring you industry-first smart technology that will transform your pharmacy’s script workflow.
Introducing MedView Flow Digital Baskets – the new way to manage scripts that is safer, easier and faster than your pharmacy’s current manual system and bridges the gap between digital processes and existing use of plastic prescription baskets.
With flexible purchase options and low setup costs, it’s easy to upgrade Medview Flow to include Digital Baskets. Find out more at www.medview.com.au/digitalbaskets
International Harmonisation of Ingredient Names (IHIN)
In 2017, the Therapeutic Goods Administration (TGA) updated over 200 active ingredient names in Australia to align with international standards, a program known as the International Harmonisation of Ingredient Names (IHIN). This introduced some new ingredient names to Australia through dual labelling, which requires both old and new names to be displayed on medicine labels.
Changes in Fred Dispense
Dual labelling will be switched off in Fred Dispense in the October update for all ingredients except those specified for ongoing dual labelling. This change means that Fred Dispense will only display new names for most generic ingredients from 1 October 2024 onwards. However, certain ingredients specified by TGA will continue with dual labelling until 30 April 2025 or indefinitely where both old and new names will be displayed.
Dual Labelling Transition Period
Dual labelling for most ingredient names ended on 30 April 2023, transitioning to displaying only the new names on labels. After this date, labels can either show the new name only or continue with both names while updates are made. Medicine sponsors have until 30 April 2026 to complete these updates. For example, medicines labelled as ‘lidocaine (lignocaine)’ will need to be updated to show ‘lidocaine’ only.
The transition period for most dual-labelled ingredients started on 1 May 2023 and ends on 30 April 2026, after which all medicines must show the sole name. Ingredient names that are changing to show new names only are found in TGA’s list of affected ingredient names marked as ‘Dual labelling - until 30 April 2023’ .
Some ingredients will have an extended dual labelling period until 2025 to give health professionals more time to adapt. Until 30 April 2025, labels must show both names for:
-
dosulepin (dothiepin) hydrochloride
-
hydroxycarbamide (hydroxyurea)
-
tetracaine (amethocaine)
-
tetracaine (amethocaine) hydrochloride
-
trihexyphenidyl (benzhexol) hydrochloride
The transition period for these starts on 1 May 2025 and ends on 30 April 2028, after which only new names will be shown.
Other ingredients will retain dual labelling indefinitely, including:
-
alimemazine (trimeprazine) tartrate
-
mercaptamine (cysteamine)
-
mercaptamine (cysteamine) bitartrate
-
mercaptamine (cysteamine) hydrochloride
-
Mycobacterium bovis (Bacillus Calmette and Guerin (BCG) strain).
Additional Community Supply Support (ACSS) Payments
According to The Pharmacy Guild, under the 8CPA, the Additional Community Supply Support Payments (ACSS) are processed automatically, so no extra work or data submission is required for claiming them. Ensure your PBS claim is closed as per usual.
See the links below for more information.
What's New in Fred Dispense
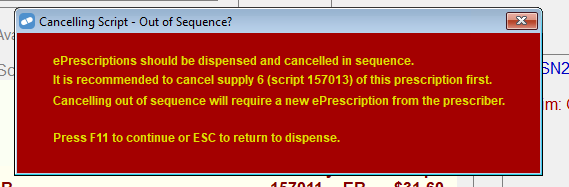
Prompt when ePrescriptions are cancelled in the wrong order
ePrescriptions should be dispensed and cancelled in sequence. This is to ensure that each supply's unique prescription identifiers for the repeat chain are re-instated to allow for potential re-dispensing of the repeat chain. If the scripts are cancelled out of sequence, then the patient will need to get a new ePrescription from the prescriber.
In order to minimise this situation from occurring, the following message will display when cancelling an ePrescription out of sequence.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site.
For Highly Specialised Drugs (HSD) items (CAR and non-CAR), please refer to the Schedule of Pharmaceutical Benefits as well as Services Australia to confirm whether the PBS item code for a HSD is eligible to be dispensed and claimed by your pharmacy type.
