June 2020
News
In this issue
| Australia’s first electronic prescription successfully dispensed in primary care |
|
| Get ready for ePrescribing |
|
| FREE Webinar - Wed 3rd June - Dispensing ePrescriptions – How it works now and into the future |
|
| End of financial year and stocktaking help |
|
| Important reminder for department stocktakes |
|
| Victoria - SafeScript naming in Fred Dispense changed to RTPM |
|
| Protecting pharmacy against data breaches |
|
| Revised Opioids PBS Listings from 1 June 2020 |
|
| This Month's PBS Changes |
|
Australia’s first electronic prescription successfully dispensed in primary care
Australia’s first paperless electronic prescription in primary care was successfully prescribed and dispensed in Victoria on the 6th May, marking a significant first step in the national delivery of electronic prescriptions. The successful electronic prescription occurred using Best Practice Software, prescription exchange service eRx Script Exchange, Fred NXT Dispense and MedView Flow.
Get ready for ePrescribing
Fred Dispense software is compliant and has been certified for ePrescriptions. Trials are underway in some pharmacies and there will be a phased approach to implement the token model. Some states or territories will be implementing ePrescribing sooner than others. For more information, see the Electronic Prescribing Factsheet provided by the Australian Digital Health Agency.
There are a number of steps you can take now to prepare for ePrescribing.
- Visit https://www.fred.com.au/what-we-do/eprescriptions/ where you can:
- View the ePrescription checklist and guide to assess your pharmacy's readiness.
- Register for MedView Flow (if you haven't already done so).
- Read about MedView Flow —pharmacy workflow management tool that brings together paper based and electronic prescriptions presented at the Scripts In counter into the one electronic workflow (or queue).
- Attend the Free Webinar on Wednesday 3rd June - Dispensing ePrescriptions.
FREE Webinar - Wed 3rd June - Dispensing ePrescriptions – How it works now and into the future
Wednesday 3rd June at 7pm (AEST) - Online
Join the pharmacists from Fred IT Group for our special two part webinar covering:
• PART A - ePrescription highlights and learnings so far (60min)
• PART B - Into the future with MedView Flow: What you need to know (30min)
End of financial year and stocktaking help
It's nearly time for End of Financial Year Reporting and Stocktaking. To assist you with these processes, refer to the following Fred Office resources from the Fred Help Centre:
- Fred Office End of Financial Year Report Recommendations
- Fred Office Stocktaking
- Fred Office External Stocktake Provider Procedure
Important reminder for department stocktakes
If you plan to do a department stocktake, make sure that all product items are assigned to a department. Otherwise, items will be missing from your stock count.
- Select Reporting > Inventory Reports > Item Pricing Details.
- Click Open Report.
- Select the No Department checkbox and then click Run Report.
- For each product item in the list, assign it to a department:
- Double click an item to display the stock card.
- Select a Department.
- Click Save and Close.
Victoria - SafeScript naming in Fred Dispense changed to RTPM
In this release of Fred Dispense, references to 'SafeScript' are now 'RTPM' to make them more generic in preparation for other jurisdictions coming online with Real Time Prescription Monitoring. SafeScript is the name of Real Time Prescription Monitoring in Victoria.
Example
Amber Notification (Before)

Amber Notification (After)
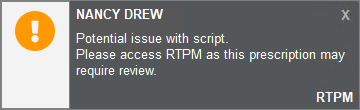
For more information, see Real Time Prescription Monitoring (RTPM) and Fred Dispense.
Protecting pharmacy against data breaches
Fred's Andrew McManus discusses the key implications for pharmacies subject to the Notifiable Data Breach Scheme and mandatory reporting of data breaches.
Revised Opioids PBS Listings from 1 June 2020
Commencing 1 June 2020, the Pharmaceutical Benefits Scheme (PBS) listings for opioid medicines is changing.
The changes include amendments to existing restriction requirements and arrangements for increased quantities and repeats. In addition, there are new Restricted Benefit listings for smaller maximum quantities of immediate release opioids, with no increased quantities or repeats, for patients requiring short-term relief of acute severe pain.
Source: http://www.pbs.gov.au/info/news/2020/05/revised-opioids-pbs-listings-from-1-june-2020
Tramadol and Paracetamol + Codeine
As part of the PBS Summary of Changes 1 June 2020, the following PBS item codes have been delisted.
Refer to page 6 Deletions - Item in the PBS Summary of Changes - June 2020 document (below) :
- 8785J PARACETAMOL + CODEINE paracetamol 500mg + codeine phosphate hemihydrate 30 mg tablet, 20 - PBS Max Qty 60
- 8611F TRAMADOL, tramadol hydrochloride 5 mg capsule, 20
Prescriptions written for Paracetamol/Codeine (Item code 8785J) prior to 1 June 2020 and claimed on or after 1 June 2020, pharmacists will need to claim under Item code 1215Y. Pharmacists will need to acknowledge the mismatch warning code presented through the PBS Online Claiming system (W162/W163) and keep sufficient information relating to the claimed prescription.
Refer to PBS Online re: Pharmacist claiming of paracetamol 500mg + codeine 30mg tablet prescriptions from 1 June
Prescriptions written for Tramadol 50mg capsules prior to 1 June 2020 and claimed on or after 1 June, pharmacists will need to claim under Item code 8455B. Where these prescriptions have 1 or 2 repeats, pharmacists will need to claim as an authority prescription for which they can enter the temporary authority prescription number 900 when dispensing Tramadol 50mg capsules under Item code 8455B. Pharmacists will need to acknowledge the mismatch warning code presented (W 150/151) and keep sufficient information relating to the claimed prescription.
Refer to PBS Online re: http://www.pbs.gov.au/info/news/2020/06/pharmacist-claiming-of-tramadol-50mg
Refer to page 6 Deletions - Item in the PBS Summary of Changes - June 2020 document (below).
Up-scheduling of Modified-Release Paracetamol Products to S3 (Pharmacist Only)
The Therapeutic Goods Administration (TGA) has made the final decision to up-schedule Modified-Release paracetamol from Schedule 2 (Pharmacy Medicine) to Schedule 3 (Pharmacist Only) commencing 1 June 2020.
Refer to https://www.tga.gov.au/changes-way-modified-release-paracetamol-products-are-supplied-questions-and-answers for more information.
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site:
Please note that the Government provides this information under embargo to Fred IT Group to enable next month’s PBS changes to be incorporated into your drug file. We have provided this information in this newsletter to prepare you for the changes, maximising patient availability/access and enabling you to adjust your stock accordingly. A condition of the embargo and in the interests of the quality use of medicines is that the information must not be released to the public or otherwise distributed prior to 1 June 2020.

