November 2017
News
In this issue
| PDL Practice Alert – Cefuroxime 125mg/5mL |
|
| Queensland Controlled Drug Reporting Is Now Weekly |
|
| DHS Update - CAR Items prescribing and dispensing combinations |
|
| This Month's PBS Changes |
|
| Running the monthly update for Fred Dispense |
|
PDL Practice Alert – Cefuroxime 125mg/5mL
Pharmacists are advised to make note that cefuroxime 125mg/5mL is available in both 70mL and 100mL units. which are similar in packaging and require differing volumes of water for reconstitution.
Awareness needs to be applied when:
- Selecting product in Dispense.
- Selecting product from shelf.
- Determining reconstitution volume.
Good dispensing practices, such as using eRx prescription scanners and ScanCheck Rx, can reduce the risk of error.
For full details of the PDL Practice Alert, refer to Look-A-Like Packaging Release.
Queensland Controlled Drug Reporting Is Now Weekly
As mentioned in previous newsletters, from 1 October 2017, S8 dispensing reports in Queensland must now be submitted weekly (for more details, please contact the Queensland Government Department of Health).
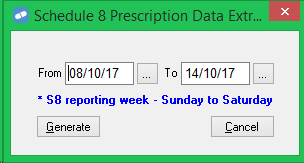
If your pharmacy is in Queensland, the Schedule 8 Prescription Data Extract report window (Reports menu > Script > S8 Script Data to Disk now displays a reminder: * S8 reporting week - Sunday to Saturday.
DHS Update - CAR Items prescribing and dispensing combinations
The Highly Specialised Drugs (HSD) Program provides access to specialised Pharmaceutical Benefits Scheme (PBS) medicines for the treatment of chronic conditions which, because of their clinical use and other special features, have restrictions on where they can be prescribed and supplied.
The PBS Online claiming system currently returns a warning to the pharmacy if there is a mismatch between the prescriber type and the approved HSD medicine claimed, however will accept the claim if the warning is acknowledged.
The Department of Human Services is making a system change effective 5 November 2017 that will reject claims made by pharmacies where they are not authorised to supply a prescription under the HSD restrictions. These changes will reduce the risk to a pharmacy that may inadvertently supply HSD items (for which they may not be entitled to a PBS payment).
The table below shows the eligible prescribing and supply combinations:
| HSD prescription type | Dispense from a Public hospital | Dispense from a Private hospital | Dispense from a Community pharmacy | Dispense from a Medical practitioner |
|---|---|---|---|---|
| HSD Public hospital (non-CAR item) | ||||
| HSD Public hospital (CAR item) | ||||
| HSD Private hospital (non-CAR item) | ||||
| HSD Private hospital (CAR item) | ||||
| HSD Community Access |
Following the change there will be new PBS Online reason codes that will reject claims where the prescribing and supply combination does not meet these rules. The reason code message will inform that the prescription cannot be claimed by your type of pharmacy.
If a prescription cannot be claimed by your type of pharmacy, the patient will need to visit a pharmacy that meets these rules or return to the prescriber for an amended prescription.
Further enquiries:
Online Technical Support Liaison
Health Systems Branch
Production Systems Delivery Centre
Department of Human Services
humanservices.gov.au/health-professionals
1300 550 115
This Month's PBS Changes
For the full Schedule of Pharmaceutical Benefits, go to the PBS website at http://www.pbs.gov.au/ where you can search the schedule by drug name.
For your convenience, we've also uploaded a printable summary of this month's important PBS changes to this site:
